Abstract
CDAR (ClinicalTrials.gov Identifier: NCT02964494), a registry for patients with Congenital Dyserythropoietic Anemia (CDA) in North America, was recently created with the goal to provide a longitudinal database and associated biorepository to facilitate natural history studies and research on the molecular pathways involved in the pathogenesis of CDAs. A 2 y.o. female patient with transfusion dependent anemia, pathologic diagnosis of Congenital Dyserythropoietic Anemia type I (CDA-I), and neurodevelopmental delay was enrolled in CDAR. Next Generation sequencing and deletion/duplication assay identified no mutations in the known CDA-associated genes, including CDAN1 and C15orf41, which are causative for CDA-I. Whole-exome sequencing for the patient and her parents (family-trio design) revealed a novel, de novo VPS4A missense variant located in the last codon of exon 8, potentially affecting splicing. VPS4A is an ATPase which, in association with the endosomal sorting complex required for transport (ESCRT), has been shown to play a critical role in cell division of HeLa cells in vitro, concentrating at the spindle poles during mitosis and at the midbody during cytokinesis. The aim of this work is to validate the pathogenetic role of the VPS4A variant for CDA and further investigate the role of VPS4A in erythroblast mitosis and cytokinesis.
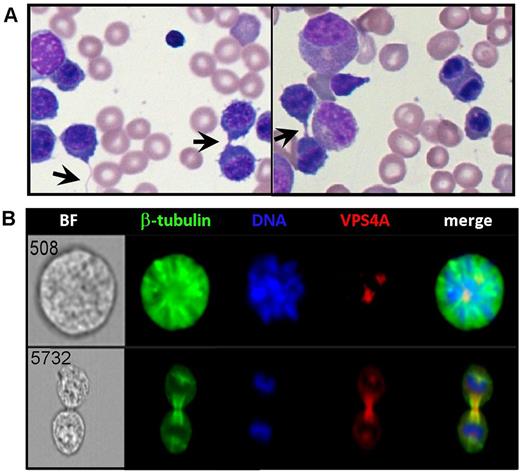
Central review of the patient's bone marrow aspirate smears revealed bi-nucleated erythroblasts in the range of 3-7%, a criterion compatible with CDA-I. However, cytoplasmic bridges were noted (arrows in Figure 1A) rather than the nuclear bridges typical of CDA-I. Immunofluorescence staining performed on erythroblasts generated ex vivo from normal CD34+ cells verified that VPS4A localizes to the spindle poles during mitosis and the midbody during cytokinesis in dividing human erythroid cells analyzed by Imaging Flow Cytometry (Figure 1B). The level of VPS4A mRNA expression in the patient's reticulocytes was evaluated by qPCR using three different sets of primers and found to be decreased by 55-70% compared to control reticulocytes and knock-down of VPS4A in normal CD34+ cells resulted in erythroid cultures enriched in binucleated cells. Induced pluripotent stem cells (iPSCs) were generated from the patient's peripheral blood mononuclear cells after the family's consent. Erythroblasts produced from these iPSCs exhibited decreased VPS4A localization at the spindle poles and midbody and fail to divide properly, frequently maintaining cytoplasmic bridges as seen in the patient's bone marrow. Additionally, flow cytometry analysis of the patient's peripheral blood cells stained with anti-CD71 for the transferrin receptor and Thiazol Orange (TO) for RNA revealed a unique cell population which is TO negative, yet CD71 positive implying that VPS4A is also involved in reticulocyte maturation, likely participating in vesicle formation and the normal exocytosis of the transferrin receptor.
VPS4A appears to play a critical role in erythroblast mitosis and cytokinesis, as well as erythrocyte maturation, and is a novel candidate gene for congenital dyserythropoietic anemia.
Figure 1. A) Binucleated erythroblasts and cytoplasmic bridges (arrows) were noted on the patient's bone marrow aspirate smears. B) VPS4A localizes at the spindle poles (upper image) and midbody (lower image) in normal human erythroblasts.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal